Participants
Vaattoegang/ Vascular access
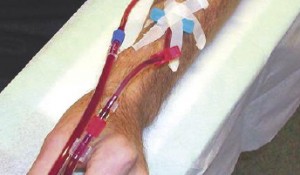
Vascular access dysfunction remains the Achilles’ heel of hemodialysis, leading to substantial morbidity and mortality. The LUMC has an extensive track record in the field of vascular access, which is organized from without our expertise center, in which nephrologists, vascular surgeons, biologists, technicians and engineers are working together. Our research includes preclinical studies on the pathophysiology of vascular access failure, epidemiological studies on the prevalence of vascular access complications, as well as the development of novel therapeutic strategies to improve the durability of vascular access conduits. The latter includes treatment with liposomal drug-formulations to target vascular inflammation, forearm exercise to enhance maturation, tissue engineered vascular grafts and novel medical devices to improve the functionality of arteriovenous access conduits.
Supported by grants from the Ministry of Economic Affairs and ZonMW (VIDI grant JI Rotmans) and in collaboration with researchers from Maastricht University (prof. Moroni), we developed a novel technique to make in situ engineered blood vessels for hemodialysis access (Geelhoed et al, Biomaterials, 2019). These vascular grafts are currently being evaluated in a clinical trial. Recently, we completed a randomized clinical trial on the efficacy of liposomal prednisolone to enhance maturation of radiocephalic fistulas (Voorzaat et al. Kidney International Reports 2020). Furthermore, we developed a new concept for a dialysis needle with the aim to reduce miscannulations (Geelhoed, Journal of Medical Devices, 2020 in press). In addition, we studied long-term outcomes of the various configurations of arteriovenous access conduits and the efficacy of interventions to maintain and restore patency (Voorzaat et al Kidney360, 2020) and we explored current strategies regarding vascular access management after transplantation (Voorzaat et al. Journal of Vascular Access, 2018). In addition, the PINCH trial (PI dr van der Bogt) is currently being performed in which the efficacy of pre-operative forearm exercise is evaluated to enhance outward remodeling of fistulas. Supported by a grant from the Health Technology program LUMC-TU Delft, we are currently developing a device that creates the option to make a dynamic AVF, a vascular access conduit that is solely functional during hemodialysis seesions (collaboration with dr.ir. T. Horeman, TU Delft).